What is Taltz (ixekizumab)?

Taltz (ixekizumab) is a biologic drug launched in 2016 that suppresses the function of an excessively increased interleukin-17A (IL-17A) substance. The drug is effective for both skin and joint symptoms caused by psoriasis.
» Click here for more information on psoriasis.
The Taltz 80mg is administered with a subcutaneous injection with the choice of an auto injector or syringe.
Patients are allowed to inject the drug themselves, and the devices are designed to be easy to administer. The auto injector device has received the Good Design Award.
Biologic treatments are approved for use by the Board of Directors after reviewed by the Biologics Review Committee of the Japanese Dermatological Association. Our clinic is included in the Japanese Dermatological Association’s list of “Facilities Approved for Use of Biologics for Psoriasis“.
Characteristics of Taltz
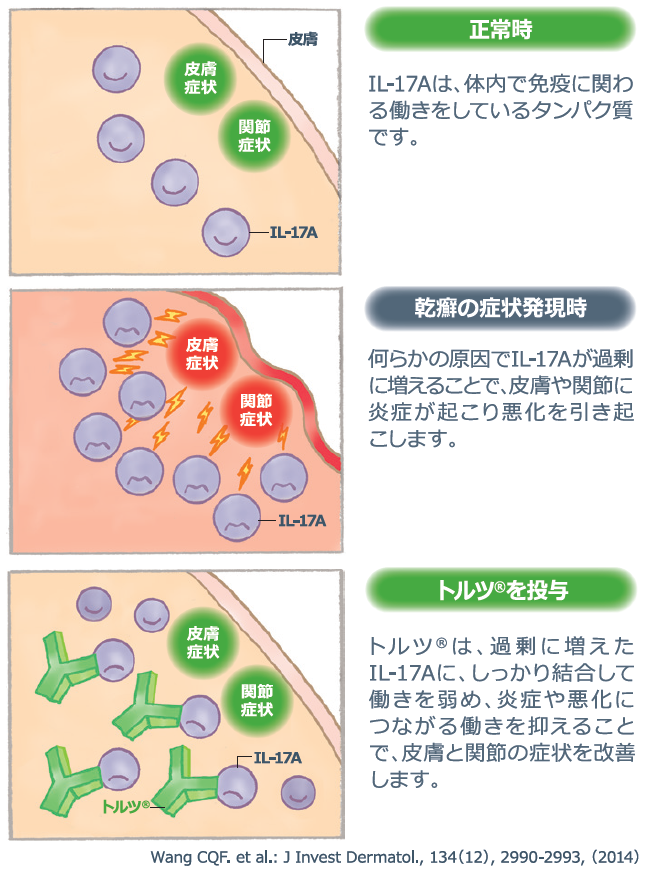
IL-17A plays an immune-related role in the body under normal conditions, but when IL-17A increases in excess for some reason, psoriasis occurs and is known to be deeply involved in causing skin and joint symptoms.
In the early stages of psoriasis, redness and itching are seen in most patients. It is difficult to distinguish from eczema because it is very similar, but as the condition progresses, the borders become more distinct and the skin becomes raised and scaly.
Taltz is a drug that selectively targets IL-17A, which is deeply involved in psoriasis. It binds firmly to the excessive amount of IL-17A and suppresses the function that leads to inflammation and the worsening of the condition. This is expected to improve skin and joint symptoms caused by psoriasis.
Patients Eligible to Use Taltz
What to check for when using Taltz
- Patients who have inadequate responses with conventional treatment methods (ointments, ultraviolet light therapy, and oral medication).
- Patients with skin symptoms that cover more than 10% of their entire body (an area equivalent to 10 palms)
- Patients with refractory skin or joint symptoms
Indications for Taltz
The following four psoriasis and arthritis conditions are eligible.
- Psoriasis vulgaris
- Psoriatic arthritis
- Psoriatic erythroderma
- Pustular psoriasis
- Ankylosing spondylitis
- Axial spondyloarthritis not meeting the x-ray criteria
Dosage and Administration Schedule
Taltz can be injected at a medical institution or self-injected by the patient.
How to use the Auto Injector
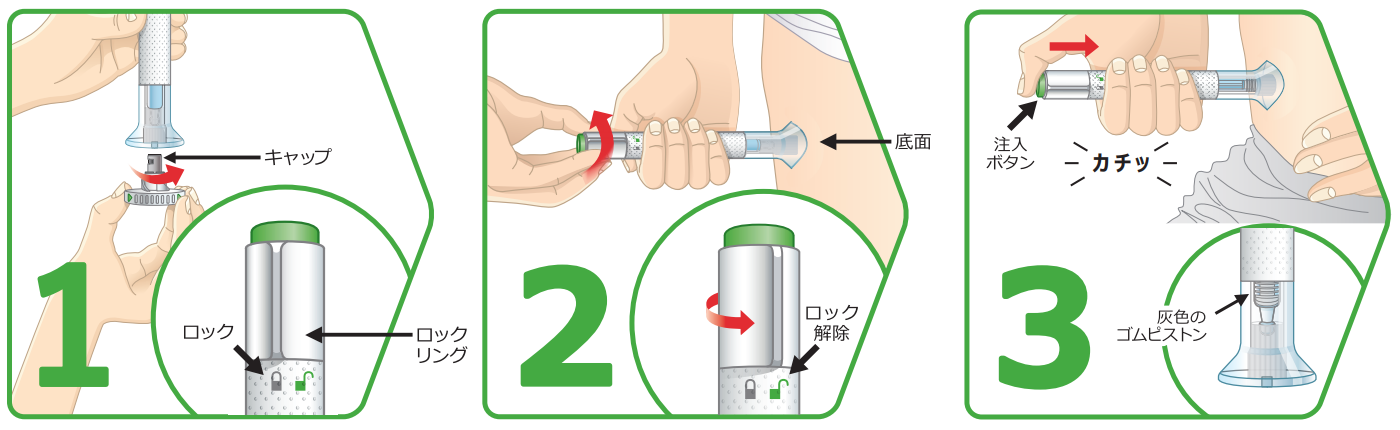
Select an administration site on the thigh, stomach or the outer side of the arm that is free of psoriasis symptoms and scars. Make sure the locking ring is in the locked position, turn and remove the cap, and secure the bottom of the cap against the skin. When the lock is released and the injection button is pushed all the way out, a loud click is heard, followed by a second loud click, and the injection is complete.
Features of Auto Injector
- The broad base adheres closely to the skin and is stable
- The design makes it difficult to see the needle
- The start and end of auto injections can be confirmed with an audible sound
Taltz Dosage Schedule
First time: Two injections are given in succession for the first time only.
Second to seventh injections: After the second injection (from 2 to 12 weeks), one injection is given at 2-week intervals.
8th and after: After 12 weeks, one injection is given at 4-week intervals.
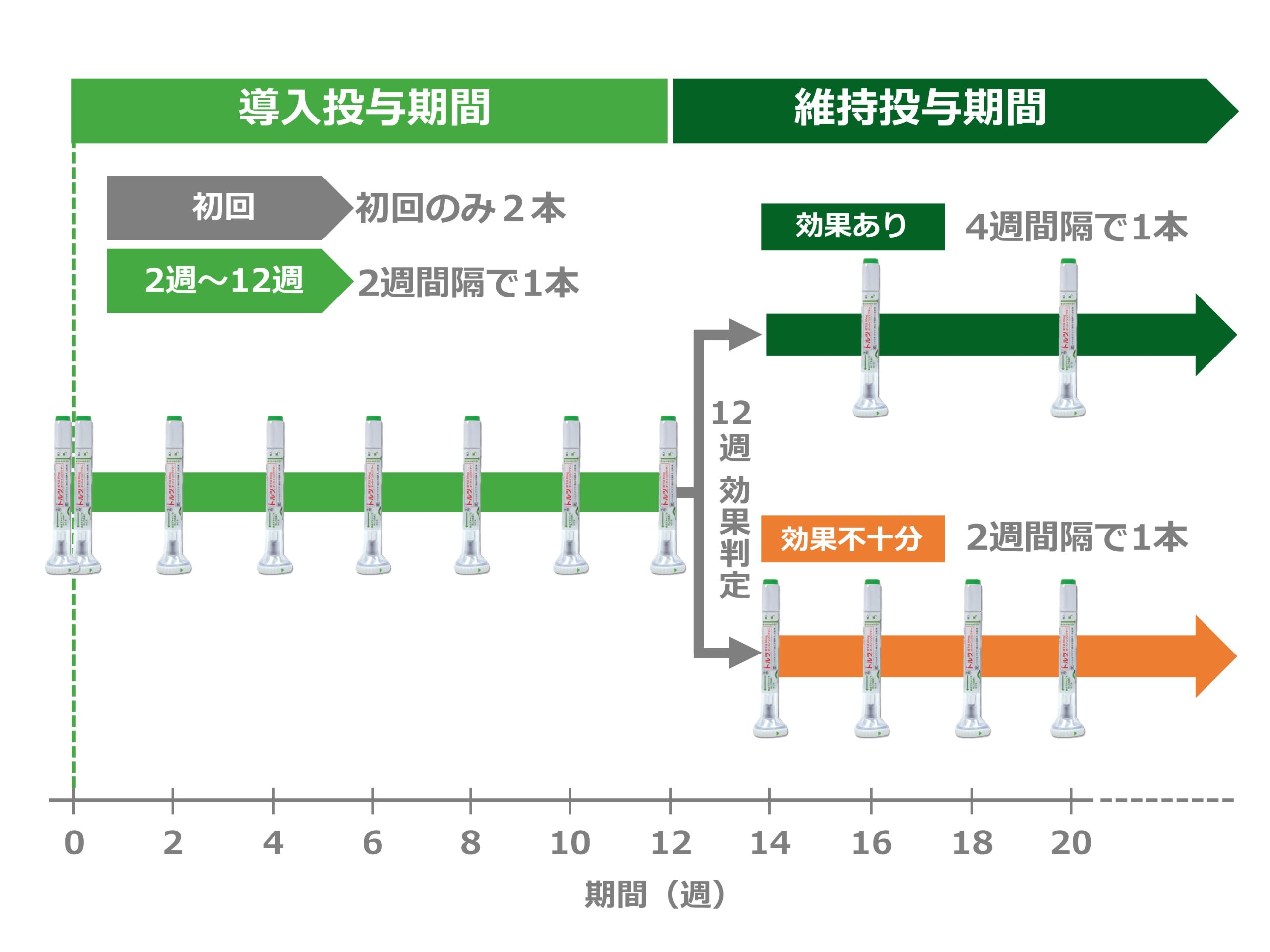
*If the effect is insufficient at 12 weeks, one injection can be given at 2-week intervals after 12 weeks.
*The number of injections and injection schedule remain the same regardless of weight or age.
*The photo shows an auto injector. The administration schedule is the same for syringes.
*The administration schedule for ankylosing spondylitis/axial spondyloarthritis that does not meet X-ray criteria is one injection per session, every 4 weeks, starting from the first injection.
If you have any questions about Taltz, we also have an inquiry office (Eli Lilly Japan K.K.) available toll-free at 0120-526-382.
Precautions for use
On the day of injection, the use of biological medication such as Talz may weaken the body’s immune function and resistance to pathogens and viruses. In your daily life, be sure to wash your hands and gargle to prevent infections such as colds and influenza, and take good care of yourself.
While using Taltz, live vaccine inoculations are not available due to the risk of causing infections.
Please contact us if you wish to receive other vaccinations.
Copayments and Drug Price
The drug fee for the Taltz subcutaneous injection 80 mg is 148,952 yen per unit for both auto injector and syringe, and the drug fee is 44,685.6 yen per unit for patients with 30% copay (drug cost only). Since the number of doses that can be administered in a month varies depending on the starting date of administration, the patient’s cost will also vary.
If medical expenses are high, you may be eligible for a reduced co-payment or a partial tax reduction.
Frequently Asked Questions
- Why is Taltz injected rather than taken?
-
Because the ingredients of Taltz are proteins, if Taltz is orally administered, it is digested in the stomach and intestines and loses its effect before reaching the site of action. In order for the active ingredients of the drug to remain stable and work properly, it is best to use it as an injectable drug.
- Does the timing or content of meals affect injections or treatment?
-
Treatment with Taltz has no effect on meals. Injections can be given before or after, so there is no need to time meals for the Taltz treatment. Also, there are no specific dietary restrictions to use Taltz.
- Do I need to undergo any tests while recei ing treatment with Taltz?
-
Routine testing is recommended during treatment with biologic medications, including Taltz, to ensure that you are being treated safely with biologic medications. In addition to periodic testing, your doctor may decide to have you undergo clinical testing to check for side effects. Please follow your physician’s instructions regarding laboratory tests.
- What should I do if I develop a fever or an infection during my Taltz treatment?
-
If you think you have an infection or if you develop any signs or symptoms of an infection after you start using Taltz, please notify your physician. You should be aware on a regular basis that the use of Taltz may have weakened your body’s ability to fight infections and made you more susceptible to illness. When consulting with your physician, please provide as much detail as possible, including what symptoms you are experiencing and when they started, and follow the physician’s advice.
- Is it safe to travel?
-
Yes, you can. However, please take care to maintain hygiene and take other precautions while using Taltz. Also, if your travel schedule is likely to overlap with the scheduled injection date, please contact us as soon as possible.